This Bacteria Will Eat Your Flesh How to Stay Safe
Although yeast and fungi play the most important roles in foodfermentation, lactic acid bacteria (LAB), a generally regarded as safe (GRAS) probiotic, is frequently included in the starter culture.

Appendix Safe Handling of Infection Public Health
Probiotic supplements are generally regarded as safe because the microorganisms they contain are identical to those found in human gastrointestinal and vaginal microflora. Guidelines on the use of probiotics in the clinical setting require periodical updates for the latest data to be included in clinical applications.

Viruses, bacteria and fungi not so yucky after all
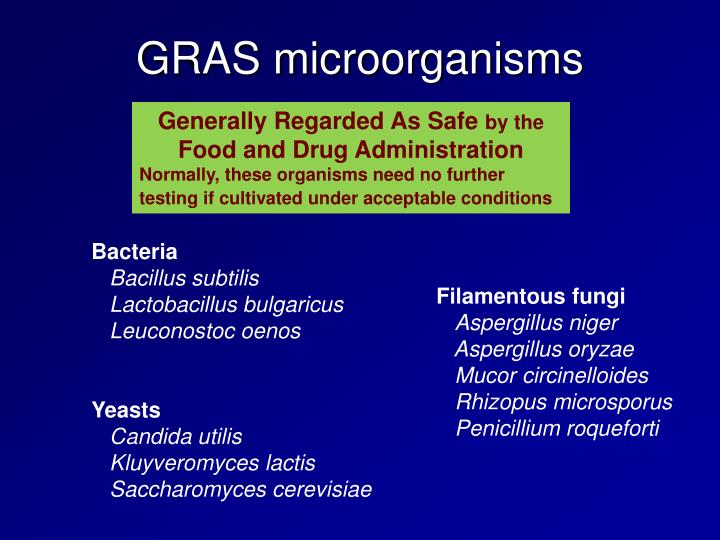
This database allows access to opinions and conclusions from 115 SCOGS reports published between 1972-1980 on the safety of over 370 Generally Recognized As Safe (GRAS) food substances.
Microfluidic device quickly classifies bacteria as safe or unsafe
The GRAS process is well suited for enzymes, given the long history of safe use, general availability of scientific data supporting enzyme safety, the generally recognized (peer-reviewed) methodology and decision trees for evaluating the safety of microbial enzymes used in food processing and in animal feed, respectively, including the
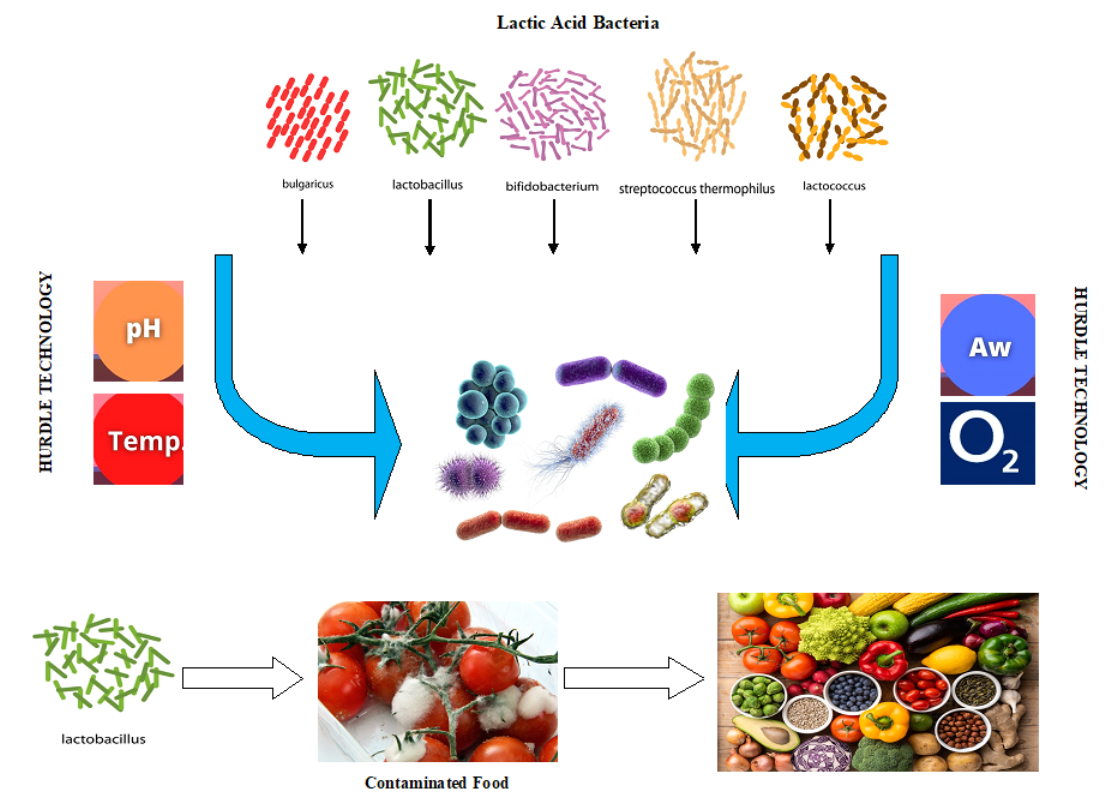
Foods Free FullText Lactic Acid Bacteria as Antimicrobial Agents
Generally recognized as safe (GRAS) is a United States Food and Drug Administration (FDA) designation that a chemical or substance added to food is considered safe by experts under the conditions of its intended use. An ingredient with a GRAS designation is exempted from the usual Federal Food, Drug, and Cosmetic Act (FFDCA) food additive tolerance requirements.

Microbes In Human Welfare Notes Class 12 Best Revision Short Notes For
Plants and microorganisms are sources of natural colours. It has been studied and investigated that fungal species such as Neurospora, Monascus. and generally regarded as safe (GRAS) food assessments. In: Food control and biosecurity. Academic Press, London, pp 543-565. Google Scholar Goodrich, William W (2010) Address to the FFDCA.
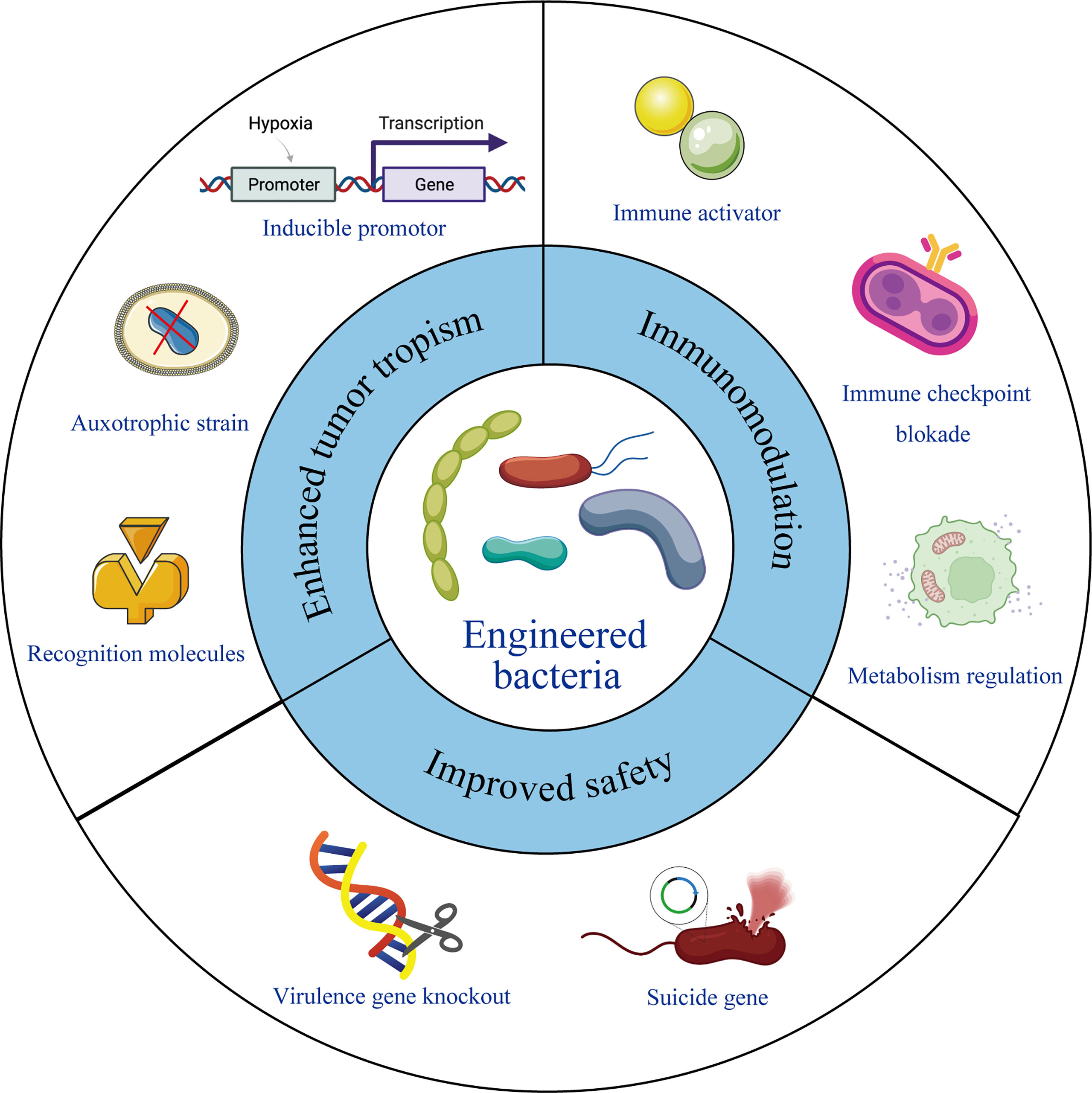
Frontiers Current Status and Future Directions of BacteriaBased
Abstract. The Generally Recognized as Safe (GRAS) process is well suited for enzymes, given the general availability of scientific data supporting enzyme safety, and the generally recognized (peer-reviewed) methodology and decision trees for evaluating the safety of microbial enzymes used in food processing and in animal feed, respectively. In.

Generally Recognized As Safe Bacteria, HD Png Download vhv
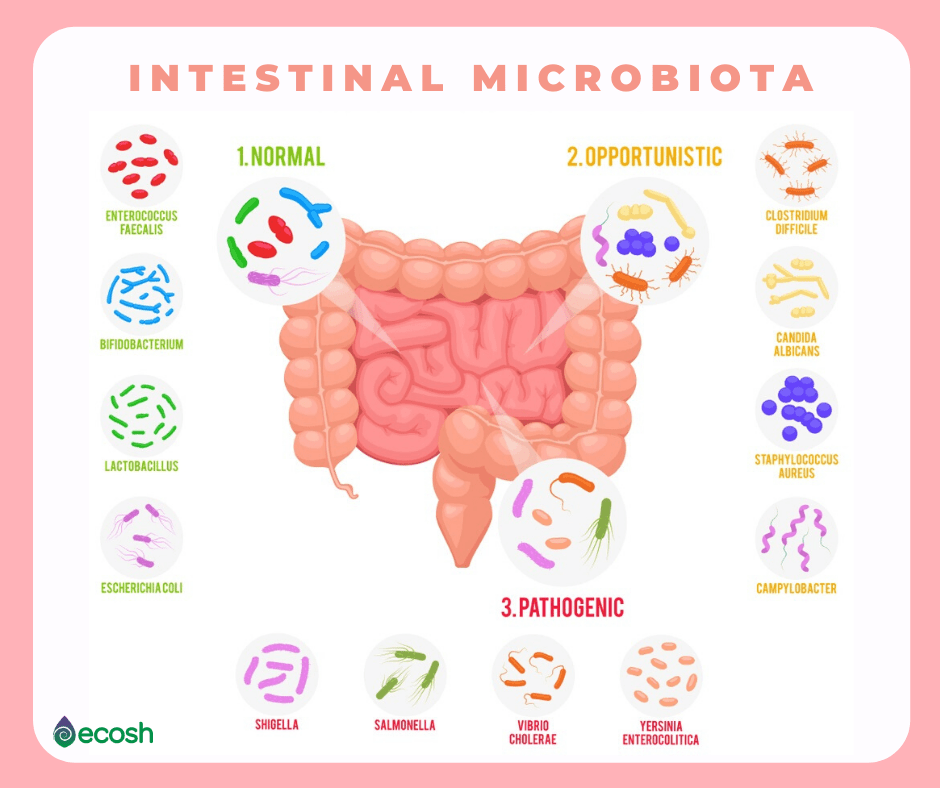
Organisms that are generally regarded as safe include lactobacilli, lactococci, Bifidobacterium, and yeast. There are other probiotic organisms, such as Enterococcus, Bacillus, and other spore-forming bacteria, as well as streptococci, that are not generally regarded as safe but have been used as probiotics. In this review, I will focus on the.

Main functions of bacteria in the gut. Bacteria benefit the host in
Section 201(s) excludes from the definition of a food additive a substance generally recognized, among experts qualified by scientific training and experience to evaluate its safety, as having been adequately shown through scientific procedures (or, in the case of a substance used in food prior to January 1, 1958, through either scientific.
PPT Microbes in service of humans PowerPoint Presentation ID6599396
Substances migrating to food from paper and paperboard products used in food packaging that are generally recognized as safe for their intended use, within the meaning of section 409 of the Act, are as follows: Alum (double sulfate of aluminum and ammonium potassium, or sodium). Aluminum hydroxide. Aluminum oleate.

Bacteria Types Chart
2.1. General Food Law: Obligation Concerns the Outcome and not the Mean. Food Including FC on the Market Must Be Safe. Food cultures (microorganisms) used directly in food production are regarded as food ingredients in the EU, a category of food ingredients with a very long history of use in a great variety of food products.

PhilDIAMOND Project 3 Development of a Safe Lactic Acid Bacteria (LAB
In addition, the possibility of production by microorganisms with GRAS status (Generally Regarded as Safe) guarantees the safety of their use due to their low toxicity and proven pathogenicity. In food processing, the ability of biosurfactants to act as emulsifiers and emulsion stabilizers allows them to be used in different concentrations, as.
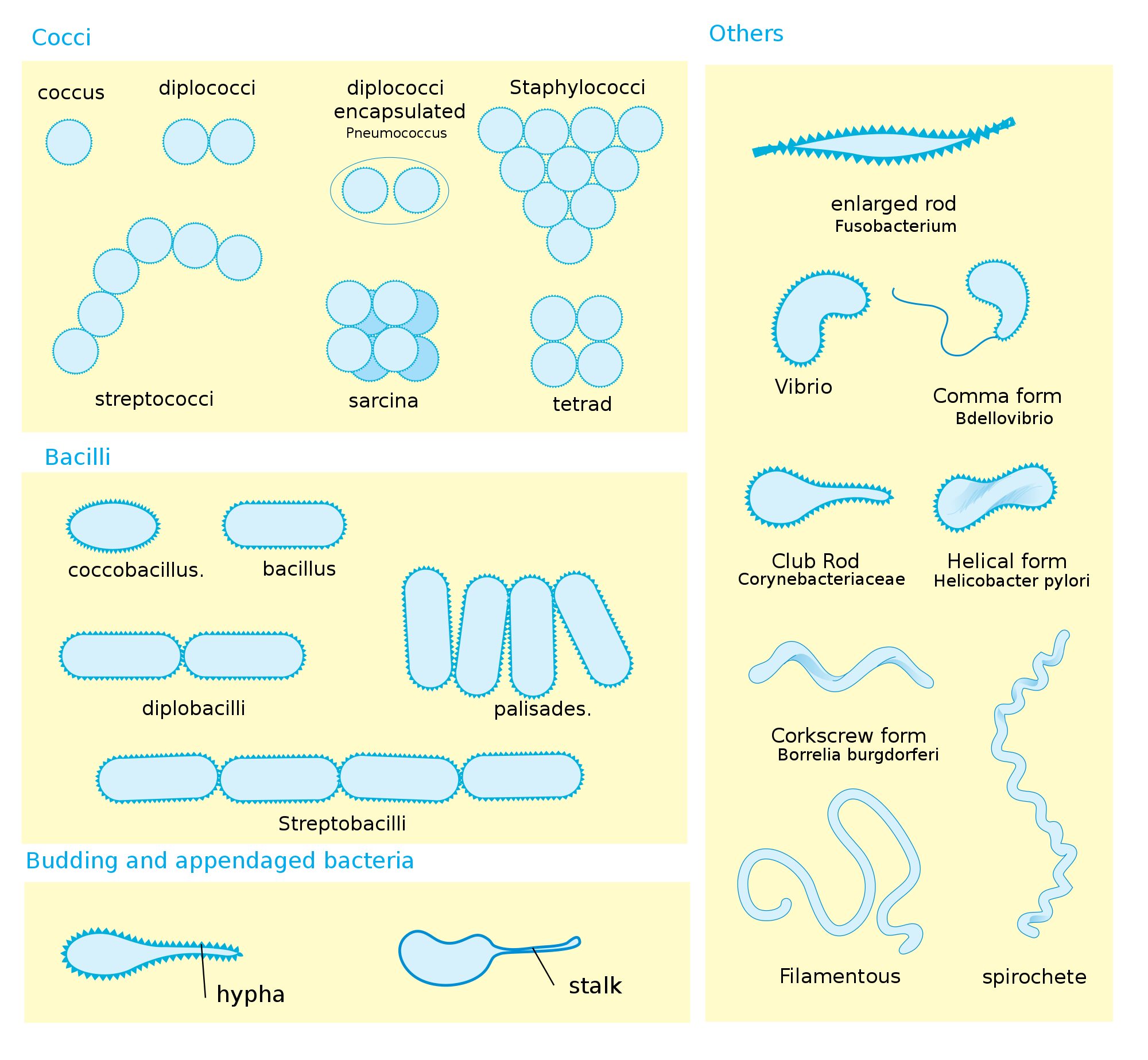
Bacteria Shape, Size, Structure and other Membrane Microbiology Notes
Enzyme preparations approved as food additives listed in in 21 CFR 173. Section in 21 CFR. Description of Enzyme Preparation. §173.110. Amyloglucosidase derived from Rhizopus niveus for use in.

Safe for Amateur Microscopy (and education) YouTube
Under sections 201 (s) and 409 of the Act, and FDA's implementing regulations in 21 CFR 170.3 and 21 CFR 170.30, the use of a food substance may be GRAS either through scientific procedures or, for a substance used in food before 1958, through experience based on common use in food. Under 21 CFR 170.30 (b), general recognition of safety.
17 TYPES OF GOOD BACTERIA The List of Most Beneficial Species of
Background. The species Lactococcus lactis belongs to the Firmicutes phylum and its strains are characterized by the production of lactic acid as the main end product of carbohydrate metabolism.L. lactis is one of the most important microorganisms in the dairy industry (Ward et al. 2002), and has "generally recognized as safe" (GRAS) status (Wessels et al. 2004).
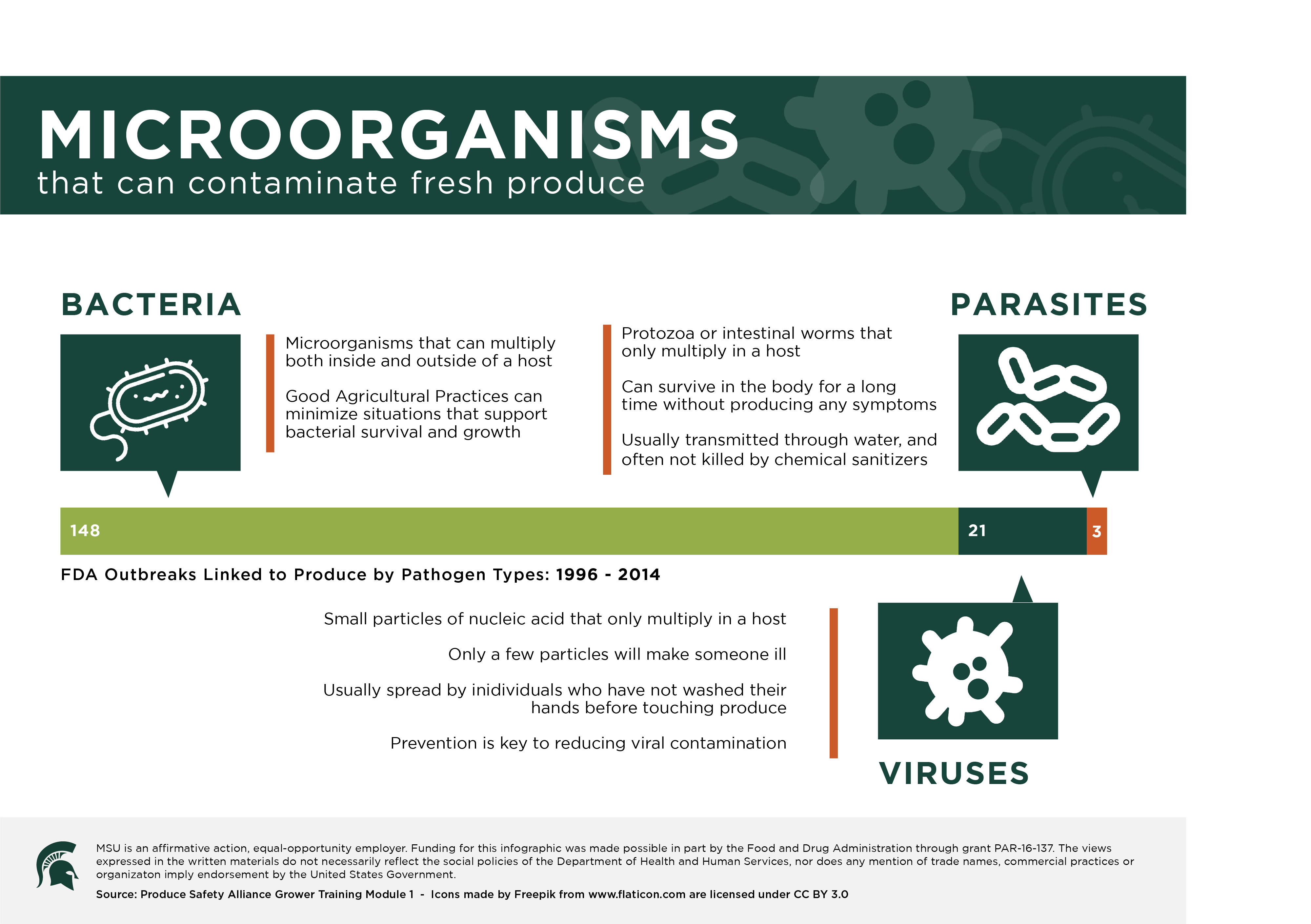
Infographic Agrifood Safety
Generally Recognized as Safe (GRAS) "GRAS" is an acronym for the phrase G enerally R ecognized A s S afe. Under sections 201 (s) and 409 of the Federal Food, Drug, and Cosmetic Act (the Act), any.
- Comprar Tablero Chapado Natural Madera Cerezo
- Aceite De Almendras Dulces En La Cara
- Fundas Asientos Coche Audi A4
- Gerencia De Atención Integrada De Ciudad Real
- Jersey Con Lazos En Las Mangas
- Maximo Parto De Una Mujer
- Amistoso España Costa De Marfil Baloncesto
- Temperatura De 38 En Adultos
- Animación 2d Y 3d Con After Effects Y Cinema 4d
- Best 3d Printer For Prosthetics