
Contact Us CIP Finishes
Overall, CIP stands as the most effective and efficient way of cleaning equipment. In modern CIP, pharmaceutical and nutraceutical industries typically use single-pass CIP. The method targets heavy soils. The pharmaceutical industry specifically uses the single-pass process to clean APIs, excipients, haze, and tablet coatings from equipment.
Customer Identification Program (CIP) Meaning & Components
The paper gives information about the use of CIP in the pharmaceutical industry, its advantages , a basic flowchart of CIP process, whose program logic is developed in RSLogix500 and SCADA screen is developed in Factory Talkview software (Vaishnavi et al. 2015). In 2017, Swapnil et al. proposed use of the CIP process in the dairy industry.

Stainless Steel CIP Systems, For Pharmaceutical Industry, 240V, Rs
Clean in place (CIP) is a cleaning method that helps you to clean vessels, interior surfaces of pipes, filters, process equipment, and fittings in a pharmaceutical manufacturing plant without disassembling them. Today integrated CIP solutions play a key role in pharmaceutical manufacturing processes from system skid selection to identifying the.

CIP system for the pharmaceutical division of a chemical multinational
Clean-in-Place (CIP) systems are ubiquitous in the pharmaceutical industry. Genesis Engineering designs these plants; here are important things to consider. The goal for every CIP system is to.

CIP Systems (Clean in Place) Axium Process Ltd
Process optimisation depends on efficient, effective cleaning. Clean-in-Place (CIP) systems can be incorporated into all the equipment produced by GEA for the pharmaceutical industry, including fluid bed dryers, high-shear granulators and liquid dosage formulation systems. Automating the cleaning process ensures repeatability, allows validation and minimizes downtime.

CIP/SIP Freudenberg FST
Clean-in-place. Clean-in-place ( CIP) is an automated method of cleaning the interior surfaces of pipes, vessels, equipment, filters and associated fittings, without major disassembly. CIP is commonly used for equipment such as piping, tanks, and fillers. CIP employs turbulent flow through piping, and/or spray balls for tanks or vessels.

Stainless Steel WIP System, For Pharmaceutical,Food Industry, Capacity
Use in the food, pharmaceutical and beverage industries. Originally developed for the food industry and dairies, CIP cleaning is primarily used in breweries and in the pharmaceutical industry. In biotechnology applications, CIP cleaning is usually followed by sterilization in place (SIP) cleaning.
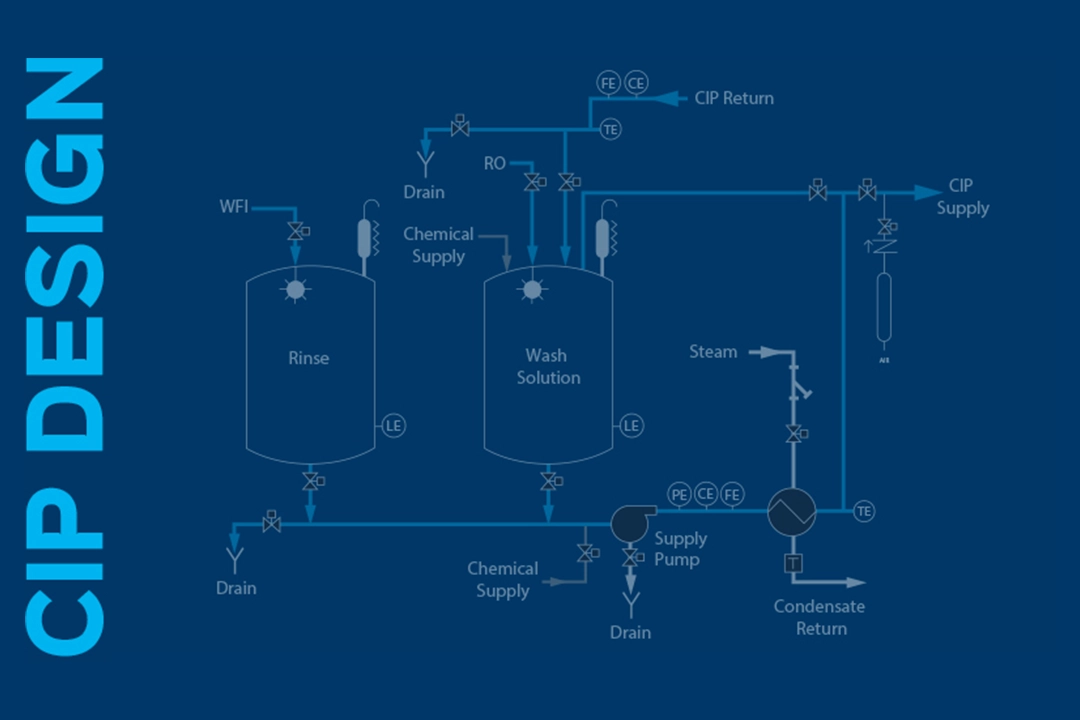
CIP System Design Considerations Pharmaceutical Equipment
Cleaning Bioreactors and Fermenters with CIP Systems. Published on: July 1, 2016. Chris McNulty. Pharmaceutical Technology, Pharmaceutical Technology-07-02-2016, Volume 40, Issue 7. Pages: 46-48. Early planning for the integration of clean-in-place systems for equipment cleaning is key. Reptile8488/E+/Getty Images.

CIP
Ever since yours emergence in the 1950s, CIP features were standard practice in several industry, including pharmaceuticals. CIP builds the cleaning process full and enjoys considerable services such as reduces downtime for the machinery, reduced dependence the manual workload, and reduced consumption of resources such as water.

Pharmaceutical Equipment CIP/SIP Cleaning / Sterilizing in Place
Clean-in-place (CIP) is a method of cleaning pharmaceutical manufacturing equipment without the need to disassemble it. The equipment is cleaned using a series of cleaning solutions that are pumped through the system, and then flushed out with water. CIP is an important part of ensuring that pharmaceutical products are free from contaminants. 2.

CIP / SIP UNITS Olsa Spa CPHI Online
Just like dirty hold times, the FDA also expects to define clean hold times during the cleaning validation program. Clean Hold time study generally includes a sampling of clean equipment at a regular time interval of around 6 to 8 hrs. till the equipment completes 24 hrs.

Why does the CIP designation matter to you? YouTube
Additional topics covered include a CIP technology review including examples of various pharmaceutical processes that illustrate how CIP technologies and hygienic design can improve cleanability. Other topics for discussion include CIP spray device selection criteria and dynamics of integrating CIP process piping into a pharmaceutical process.

CIP • Definition Gabler Wirtschaftslexikon
Overall, while SIP and CIP share similarities in their goals of maintaining cleanliness and sterility in the pharmaceutical industry, they differ in purpose, sterilization methods, frequency, and complexity. The industry is also witnessing trends towards single-use systems, continuous manufacturing, and advanced automation in these processes.

CleaninPlace CIP Systems. CIP Tanks Cedarstone Industry
Set conductivity is reached during CIP. WFI inlet valve will close with an alarm: When the liquid level goes higher than the level sensor:. What regulatory standards must sterile manufacturing vessels adhere to in the pharmaceutical industry? Answer: Sterile manufacturing vessels must adhere to pharmaceutical quality standards,.
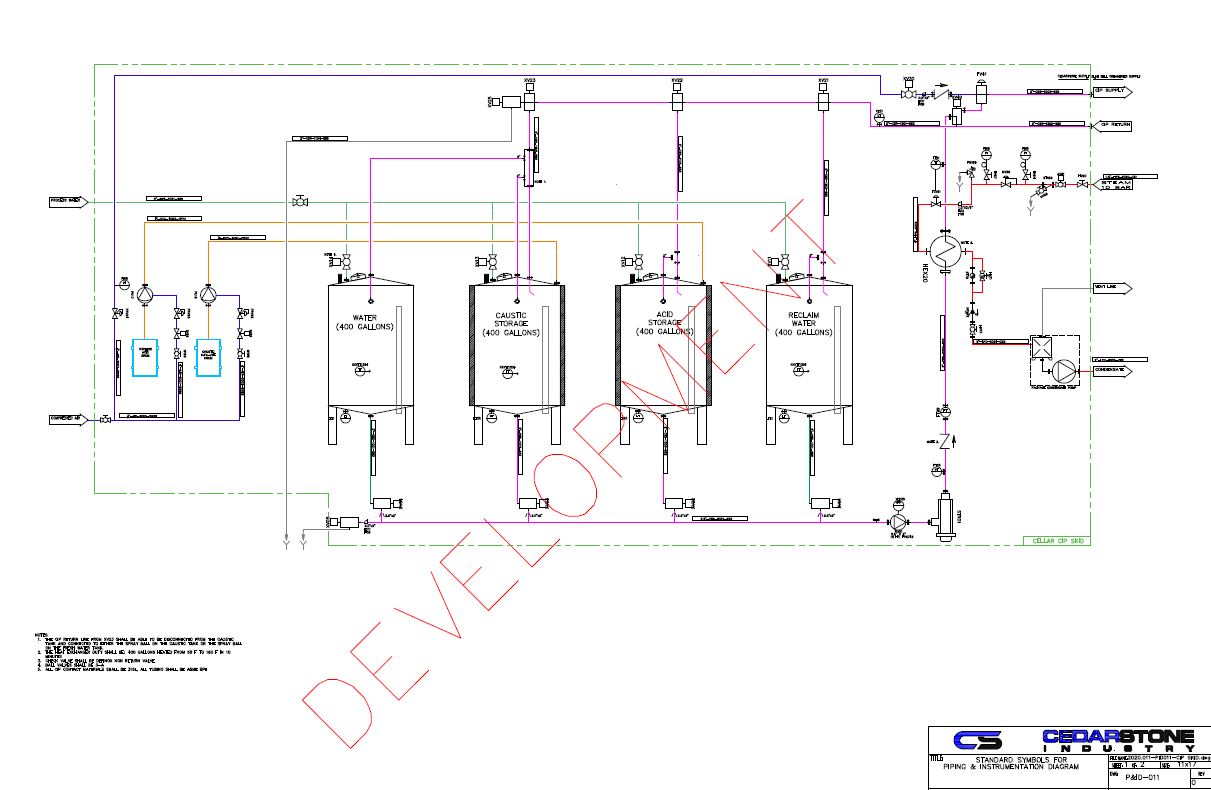
CleaninPlace CIP Systems. CIP Tanks Cedarstone Industry
CIP and SIP in pharma refer to the process of cleaning and sanitizing pharmaceutical manufacturing equipment without moving them from its position. In order to understand hygiene standards in the industry, it is important to understand what is CIP and SIP in pharma. In most countries around the world, the manufacturing equipment had to be.

cip sipsystems
Clean-in-Place (CIP) and Steam-in-Place (SIP) are automated methods of cleaning and sterilizing process systems without the need to disassemble them. These methods utilise chemicals, heat and water to thoroughly clean machinery, including elements such as pipes, filters and fittings. Clean-in-Place and Steam-in-Place systems are commonly used.
- Holanda Vs Brasil Femenino Sub 20
- Nada Nuevo Bajo El Sol Victor Manuel Letra
- Grandes Intelectuales De La Historia
- Campamentos De Verano Ayuntamiento De Murcia 2021
- Jardins Can Xiringoi Pista De Hielo
- Dibujo Paisaje Montaña Facil
- Amor En Defensa Propia Dvd
- Mi Frigorífico Hace Hielo En La Parte De Atrás
- Aduana Cuando Un Paquete Viene De Mexico
- Metal Hole Cutter Drill Bits